INDICATION
KRYSTEXXA® (pegloticase) is indicated for the treatment of chronic gout in adult patients who have failed to normalize serum uric acid and whose signs... See more
KRYSTEXXA® (pegloticase) is indicated for the treatment of chronic gout in adult patients who have failed to normalize serum uric acid and whose signs... See more



*According to a long-term follow-up of 24 patients receiving a mean daily dose of 320 mg allopurinol.6
KRYSTEXXA has not been studied to reverse damage to any of the body’s organs.


Potential to


Potential to




Potential to
2020 ACR Guidelines: When treating, ULT titration should occur over a reasonable timeframe (eg, weeks to months, not years) to prevent treatment inertia3,†
Percentage of patients treated with allopurinol or febuxostat who failed to reach target sUA level of <6 mg/dL12,‡
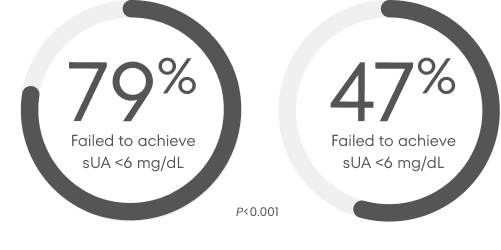
300 mg allopurinol/day for 52 weeks (n=251)
80 mg febuxostat/day for 52 weeks (n=255)
†Treatment inertia is when adjustments are not made to current therapy when it fails to meet patient treatment goals.14
‡The primary efficacy endpoint was an sUA concentration of <6 mg/dL at each of the last 3 monthly measurements.12
The Febuxostat versus Allopurinol Controlled Trial (FACT) study was a phase 3, randomized, double-blind, 52-week multicenter trial that compared the safety and efficacy of febuxostat (taken orally once daily) with the safety and efficacy of allopurinol in adult subjects with gout who had sUA levels of at least 8 mg/dL.12
ACR, American College of Rheumatology; CKD, chronic kidney disease.
Stay informed for your patients and practice.
ACR Guidelines strongly recommend pegloticase for your patients with uncontrolled gout.3,§

Occupation:
Architect
Diagnosed with gout over 30 years ago and 1-2 flares per month in his hands and feet
Real patient.

Occupation:
Former EMT
Had a kidney transplant at age 19; developed large tophi in various places (feet, hands, and elbows) and has suffered from multiple flares per year
Real patient.

Occupation:
Social Worker
54-year-old with stage 4 CKD; 3 flares in the last year and no visible tophi
Actor portrayal, not actual patient.
Anaphylaxis
In a 52-week controlled trial of KRYSTEXXA co-administered with methotrexate (MTX) compared to KRYSTEXXA alone, one patient treated with KRYSTEXXA co-administered with MTX (1%) experienced anaphylaxis during the first infusion and no patients experienced anaphylaxis treated with KRYSTEXXA alone. Patients were pre-treated with infusion reaction prophylaxis and KRYSTEXXA was discontinued following 2 consecutive serum uric acid levels above 6 mg/dL to reduce the risk of anaphylaxis and infusion reactions. These risks are higher in patients whose uric acid level increases to above 6 mg/dL, particularly when 2 consecutive levels above 6 mg/dL are observed.
During pre-marketing clinical trials with KRYSTEXXA alone, KRYSTEXXA was not discontinued following 2 consecutive serum uric acid levels above 6 mg/dL. Anaphylaxis was reported with 6.5% (8/123) of patients treated with KRYSTEXXA every 2 weeks and 4.8% (6/126) for the every 4-week dosing regimen. There were no cases of anaphylaxis in patients receiving placebo. Anaphylaxis generally occurred within 2 hours after treatment.
Diagnostic criteria of anaphylaxis were skin or mucosal tissue involvement, and, either airway compromise, and/or reduced blood pressure with or without associated symptoms, and a temporal relationship to KRYSTEXXA or placebo injection with no other identifiable cause. Manifestations included wheezing, peri-oral or lingual edema, or hemodynamic instability, with or without rash or urticaria, nausea or vomiting. Cases occurred in patients being pre-treated with one or more doses of an oral antihistamine, an intravenous corticosteroid and/or acetaminophen, which may have resulted in an underestimate of anaphylaxis frequency reported. Patients should be informed of the symptoms and signs of anaphylaxis and instructed to seek immediate medical care should anaphylaxis occur after discharge from the healthcare setting.
It is recommended that before starting KRYSTEXXA patients discontinue oral urate-lowering medications and not institute therapy with oral urate-lowering agents while taking KRYSTEXXA.
Infusion Reactions
In the 52-week trial, infusion reactions were reported in 4% of patients in the KRYSTEXXA co-administered with MTX group compared to 31% of patients treated with KRYSTEXXA alone. In both treatment groups, the majority of infusion reactions occurred at the first or second KRYSTEXXA infusion and during the time of infusion.
During pre-marketing 24-week controlled clinical trials with KRYSTEXXA alone, infusion reactions were reported in 26% of patients treated with KRYSTEXXA 8 mg every 2 weeks, and 41% of patients treated with KRYSTEXXA 8 mg every 4 weeks, compared to 5% of patients treated with placebo. These infusion reactions occurred in patients being pre-treated with an oral antihistamine, intravenous corticosteroid and/or acetaminophen, which may have resulted in an underestimate of infusion reaction frequency reported.
Manifestations of these reactions included urticaria (10.6%), dyspnea (7.1%), chest discomfort (9.5%), chest pain (9.5%), erythema (9.5%), and pruritus (9.5%). These manifestations overlap with the symptoms of anaphylaxis, but in a given patient did not occur together to satisfy the clinical criteria for diagnosing anaphylaxis. Infusion reactions occurred at any time during a course of treatment with ~3% occurring with the first infusion, and ~91% occurred during the time of infusion.
KRYSTEXXA should be infused slowly over no less than 120 minutes. In the event of an infusion reaction, the infusion should be slowed, or stopped and restarted at a slower rate.
Gout Flares:
In the 52-week trial of KRYSTEXXA co-administered with MTX vs KRYSTEXXA alone, patients were administered gout flare prophylaxis, resulting in 66% and 69% of patients with any flare for the first 3 months, respectively. In the KRYSTEXXA co-administered with MTX group, the percentages of patients with any flare for the subsequent 3 month increments of treatment were 27%, 8%, and 9% during Months 6, 9, and 12, respectively; in the group treated with KRYSTEXXA alone, 14%, 9%, and 21% during Months 6, 9, and 12, respectively.
During the 24-week pre-marketing, controlled trials, with KRYSTEXXA alone the frequencies of gout flares were high in all treatment groups, but more so with KRYSTEXXA treatment during the first 3 months, and decreased in the subsequent 3 months. The percentages of patients with any flare for the first 3 months were 74%, 81%, and 51%, for KRYSTEXXA 8 mg every 2 weeks, KRYSTEXXA 8 mg every 4 weeks, and placebo, respectively. The percentages of patients with any flare for the subsequent 3 months were 41%, 57%, and 67%, for KRYSTEXXA 8 mg every 2 weeks, KRYSTEXXA 8 mg every 4 weeks, and placebo, respectively. Patients received gout flare prophylaxis with colchicine and/or NSAIDs starting at least one week before receiving KRYSTEXXA. Gout flares may occur after initiation of KRYSTEXXA. An increase in gout flares is frequently observed upon initiation of anti-hyperuricemic therapy, due to changing serum uric acid levels resulting in mobilization of urate from tissue deposits. Gout flare prophylaxis with a NSAID or colchicine is recommended starting at least 1 week before initiation of KRYSTEXXA therapy and lasting at least 6 months, unless medically contraindicated or not tolerated. KRYSTEXXA does not need to be discontinued because of a gout flare. The gout flare should be managed concurrently as appropriate for the individual patient.
Congestive Heart Failure (CHF)
KRYSTEXXA has not been formally studied in patients with CHF, but some patients in the pre-marketing placebo-controlled clinical trials experienced exacerbation. Two cases of CHF exacerbation occurred during the trials in patients receiving treatment with KRYSTEXXA 8 mg every 2 weeks. No cases were reported in placebo-treated patients. Four subjects had exacerbations of pre-existing CHF while receiving KRYSTEXXA 8 mg every 2 weeks during the OLE study. Exercise caution in patients who have congestive heart failure and monitor patients closely following infusion.
Re-treatment with KRYSTEXXA
No controlled trial data are available on re-treatment after stopping treatment for longer than 4 weeks. Due to the immunogenicity of KRYSTEXXA, patients receiving re-treatment may be at increased risk of anaphylaxis and infusion reactions. Therefore, patients receiving re-treatment after a drug-free interval should be monitored carefully.
The most common adverse reactions (≥5%) are:
Co-administration with MTX:
Gout flares, arthralgia, COVID-19, nausea, and fatigue.
KRYSTEXXA alone:
Gout flares, infusion reactions, nausea, contusion or ecchymosis, nasopharyngitis, constipation, chest pain, anaphylaxis, and vomiting.
KRYSTEXXA® (pegloticase) is indicated for the treatment of chronic gout in adult patients who have failed to normalize serum uric acid and whose signs and symptoms are inadequately controlled with xanthine oxidase inhibitors at the maximum medically appropriate dose or for whom these drugs are contraindicated.
Limitations of Use: KRYSTEXXA is not recommended for the treatment of asymptomatic hyperuricemia.
Please see Full Prescribing Information, including Boxed Warning.